Reports to: Plant Manager
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for an Operator II to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
Reporting to Plant Manager, this position will have direct responsibility for the processing of parts through various functions within the Department. TOMZ manufactures parts via high precision metal machining, finishing, assembly, and anodize processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, prepare materials to be processed through various operations, and perform basic visual and dimensional inspections on completed components.
Essential Functions
- Must be knowledgeable of, and adhere to, the TOMZ Quality Management System.
- Prepare and process components through various metal finishing operations. Ex. include operation of manual sand blasting equipment, fixturing and operation of laser marking equipment, fixturing and operation of laser welding equipment, loading and un-loading heat treat-furnace, etc.
- Perform visual and basic dimensional inspections on completed components, as required using micrometers, calipers, microscope, etc.
- Completes and compiles necessary documentation related to Quality Inspection standards.
- Ensure proper material control, identification and traceability is maintained for conforming and nonconforming material through the manufacturing processes.
- Support Quality Best Practices and GDP/GMP continuous improvement efforts.
- Utilize and navigate ERP and QMS systems to ensure inspection and traceability activities are properly documented and controlled, as needed.
- Must follow occupational Safety and Health requirements including Personal Protective Equipment (PPE) guidance and rules.
- Other duties and responsibilities are assigned.
Qualifications:
Education
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
Experience
- Minimum of 2-5 years’ experience in a regulated manufacturing environment.
Preferred Skills/Qualifications
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g. Aerospace, Defense, Pharmaceutical, etc.)
- Knowledge of and experience with GMP/ISO standards.
- Strong background concerning the usage and care of mechanical metal finishing equipment and processes.
- Ability to use basic hand-held tools to perform preventive maintenance activities.
- Ability to effectively read and understand blueprints, specifications and procedures.
- Maintains accuracy and attention to detail at all times and completes tasks in a timely manner.
- “Hands-on” self-starter with ability to work both independently and as part of a team.
- Ability to work with a variety of functional areas, including R&D, Manufacturing, and QA, as required, accomplishing results with minimal guidance.
- Strong verbal and written English language communication skills.
Physical Demands
While performing the duties of this job, the employee is frequently required to sit, talk and/or hear, and/or use hands to finger, handle, or touch objects, tools, or controls. The employee is frequently required to stand, and/or walk. The employee must occasionally lift and/or move up to 50 pounds while moving components or fixtures/racking. The mental and physical requirements described here are representative of those that must be met by an individual to successfully perform the essential functions of this position.
Monday- Friday (8 hour shift)
Reports to: Plant Manager TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for an Operator II to join our organization. TOMZ offers ...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Quality Inspector to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
Reporting to Department Manager, this position will have direct responsibility for the quality control functions within the Department. TOMZ manufactures parts via high precision metal machining, assembly, and anodize processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation and/or perform inspections on machined components by utilizing conventional layout inspection techniques and equipment.
Essential Functions
- Must be knowledgeable of, and adhere to, the TOMZ Quality Management System.
- Perform inspections on machined components, as required, utilizing conventional visual and layout inspection techniques and equipment. Ex. include microscopes, calipers, micrometers and gages.
- Execute Incoming, First Piece, FAI, In-process, and Final Inspection, as assigned.
- Completes and compiles necessary documentation related to Quality Inspection standards.
- Ensure proper material control, identification and traceability is maintained for conforming and nonconforming material through the manufacturing processes.
- Accurately review and ensure Good Document Practices (GDP) are upheld for Routers/Inspection Plans/Device History Records (DHRs) throughout the manufacturing process and in preparation for final lot release/shipment.
- Support Quality Best Practices and GDP/GMP continuous improvement efforts.
- Utilize and navigate ERP and QMS systems to ensure inspection and traceability activities are properly documented and controlled, as needed.
- Utilize the ERP system to prioritize and support product final acceptance and shipment, as needed.
- Generate Packing Slips and Certificates of Compliance (C of C) in accordance with TOMZ and/or customer requirements.
- Support physical and/or electronic record retention.
- Must follow occupational Safety and Health requirements including Personal Protective Equipment (PPE) guidance and rules.
- Other duties and responsibilities are assigned.
Qualifications:
Education
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
Experience
- Minimum of 0-2 years’ experience in a regulated manufacturing environment.
Preferred Skills/Qualifications
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g. Aerospace, Defense, Pharmaceutical, etc.)
- Knowledge of and experience with GMP/ISO standards.
- Strong background concerning the usage and care of mechanical measurement equipment, tools and hand-held tools including micrometers, calipers, dial indicators, height gages, pin gages, etc.
- Ability to effectively read and understand blueprints, specifications and procedures.
- Maintains accuracy and attention to detail at all times and completes tasks in a timely manner.
- Knowledge of dimensional, visual and mechanical inspection processes.
- Strong verbal and written English language communication skills.
- Competency with Microsoft Office (i.e. Outlook, Word, Excel, and PowerPoint).
Physical Demands
While performing the duties of this job, the employee is frequently required to sit, talk and/or hear, and/or use hands to finger, handle, or touch objects, tools, or controls. The employee is occasionally required to stand, and/or walk. The employee must occasionally lift and/or move up to 20 pounds while moving files or small packages. The mental and physical requirements described here are representative of those that must be met by an individual to successfully perform the essential functions of this position.
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Quality Inspector to join our organization. TOMZ offers competitive compensat...
TOMZ Corporation is looking to hire a Sr. Quality Engineer – Sustained Manufacturing to join our organization. A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and excellent benefits. Those include 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
This position will be responsible for the activities associated with Quality Sustained Manufacturing of commercial product and successful transfer of Development programs to standard production, through leadership, coaching and technical guidance of cross-functional Engineering teams. This role will be expected to provide input on, and contribute to, development and implementation of Quality System improvement strategies and approaches. Fast-paced work environment.
Essential Functions
- Initiate and/or consult in the development and/or update of Risk Management FMEA documentation in cooperation with cross-functional engineering teams per project requirements.
- Support operational and process qualifications for customer manufacturing processes in cooperation with cross-functional engineering teams.
- Provide direction in implementing and continuously improving Incoming Inspection, First Article Inspection, In-Process and Final Product Inspection plans.
- Ensure the continuous efficiency and improvement of Test/Inspection Methods
- Develop and justify appropriate sampling plans with characterization of test/inspection methods and acceptance criteria.
- Apply sound, systematic problem-solving methodologies in identifying, prioritizing, communicating, and resolving quality issues, including CAPAs, Complaints and NCRs.
- Provide statistical data / trending analysis on Complaints, NCRs and other quality metrics to drive escalation and appropriate Corrective Actions to mitigate future recurrences of nonconformances.
- Conduct audits for internal manufacturing processes to ensure compliance with work instructions, summarize findings, outline opportunities for improvement, and proactively identify potential non-conformances.
- Assist in qualification activities associated with supplier/vendor design characterization requirements for projects, to include audits and assessments.
- Provide support for the compliance of Quality System support elements (QM 'feeder' systems) for the site QMS and the tracking & reporting of associated metrics as required.
- Support and enforce Quality Best Practices and GDP/GMP continuous improvement efforts.
- Support site customer-requested auditing activities (Customer and Regulatory Agencies).
- Ensure compliance of all site personnel to site-level QMS and functional training requirements.
- Be able to read and understand blueprints.
- Other duties and responsibilities as assigned.
Qualifications:
Education
- Minimum 4-Year degree or equivalent of directly transferrable industry work experience (Engineering or Quality discipline preferred).
Experience
- Minimum of 7+ years’ experience in a regulated manufacturing environment.
Qualifications
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g. Aerospace, Defense, Pharmaceutical, etc.)
- Knowledge and experience with external standards: ISO 900/9001, ISO 13485, and 21CFR 820, EU MDR, especially pertaining to product development, design controls, good manufacturing practices, supplier qualification, auditing, quality control (GD&T, Nonconforming Materials, MRB), Corrective and Preventive Actions, and customer complaints).
- Experience in effectively supporting audits by customers and external regulatory agencies.
- Strong verbal and written English language communication skills.
- Competency with Microsoft Office (i.e. Outlook, Word, Excel, and PowerPoint).
- “Hands-on” self-starter with ability to work both independently and as part of a team.
- Ability to work with a variety of functional areas, including R&D, Manufacturing, and QA.
- Ability to independently drive all items in Essential Functions.
- Lead and mentor new employees.
Preferred Skills
- Advanced Post-Secondary Education/Training/Certification coursework
- Quality certification(s) (e.g. ASQ CQE, QCI, etc.).
Physical Demands
While performing the duties of this job, the employee is frequently required to sit, talk and/or hear, and/or use hands to finger, handle, or touch objects, tools, or controls. The employee is occasionally required to stand, and/or walk. The employee must occasionally lift and/or move up to 10 pounds while moving files or small packages. The mental and physical requirements described here are representative of those that must be met by an individual to successfully perform the essential functions of this position.
TOMZ Corporation is looking to hire a Sr. Quality Engineer – Sustained Manufacturing to join our organization. A leader in manufacturing of devices and components for major medical device companies,...
TOMZ Corporation is in search of a Quality Inspector to join our team! A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and excellent benefits. Some of those include 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
Reporting to Department Manager, this position will have direct responsibility for the quality control functions within the Department. TOMZ manufactures parts via high precision metal machining, assembly, and anodize processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation and/or perform inspections on machined components by utilizing conventional layout inspection techniques and equipment.
Essential Functions
- Perform further complex inspections on machined components utilizing methods such as comparators, CMM and/or various manual and automated techniques.
- Perform and provide training assistance for inspections on machined components, as required, utilizing conventional visual and layout inspection techniques and equipment. Ex. include microscopes, calipers, micrometers and gages.
- Execute Incoming, First Piece, FAI, In-process, and Final Inspection, as assigned.
- Completes and compiles necessary documentation related to Quality Inspection standards.
- Ensure proper material control, identification and traceability is maintained for conforming and nonconforming material through the manufacturing processes.
- Accurately review and ensure Good Document Practices (GDP) are upheld for Routers/Inspection Plans/Device History Records (DHRs) throughout the manufacturing process and in preparation for final lot release/shipment.
- Support Quality Best Practices and GDP/GMP continuous improvement efforts.
- Utilize and navigate ERP and QMS systems to ensure inspection and traceability activities are properly documented and controlled, as needed.
- Utilize the ERP system to prioritize and support product final acceptance and shipment, as needed.
- Generate Packing Slips and Certificates of Compliance (C of C) in accordance with TOMZ and/or customer requirements.
- Support physical and/or electronic record retention.
- Must follow occupational Safety and Health requirements including Personal Protective Equipment (PPE) guidance and rules.
- Other duties and responsibilities as assigned.
Qualifications:
Education
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
Experience
- Minimum of 3-5 years’ experience in a regulated manufacturing environment.
Qualifications
- Regulated manufacturing industry experience (e.g. Aerospace, Defense, Pharmaceutical, etc.)
- Ability to effectively read and understand blueprints, specifications, and procedures.
- Knowledge of dimensional, visual and mechanical inspection processes.
- Strong background concerning the usage and care of mechanical measurement equipment, tools and hand-held tools including micrometers, calipers, dial indicators, height gages, pin gages, etc.
- Maintains accuracy and attention to detail at all times and completes tasks in a timely manner.
- “Hands-on” self-starter with ability to work both independently and as part of a team.
- Strong verbal and written English language communication skills.
- Competency with Microsoft Office (i.e. Outlook, Word, Excel, and PowerPoint).
- Ability to work with a variety of functional areas, including R&D, Manufacturing, and QA, as required, accomplishing results with minimal guidance.
Preferred Skills
- Quality certification(s) (e.g. ASQ CQT, QCI, etc.).
- Class I, II and/or III Medical Device manufacturing experience.
- Knowledge of and experience with GMP/ISO standards.
Physical Demands
While performing the duties of this job, the employee is frequently required to sit, talk and/or hear, and/or use hands to finger, handle, or touch objects, tools, or controls. The employee is occasionally required to stand, and/or walk. The employee must occasionally lift and/or move up to 20 pounds while moving files or small packages. The mental and physical requirements described here are representative of those that must be met by an individual to successfully perform the essential functions of this position.
TOMZ Corporation is in search of a Quality Inspector to join our team! A leader in manufacturing of devices and components for major medical device companies, TOMZ offers competitive compensation and ...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Quality Inspector to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
The successful candidate will be expected to perform inspections on machined components by utilizing conventional layout inspection techniques and equipment.
Technical Skills
- Inspection Techniques: Proficiency in using various inspection methods like comparators, CMMs, microscopes, calipers, micrometers, and gauges.
- Documentation: Ability to accurately complete and compile quality inspection documents according to established standards.
- Material Control: Understanding of proper material identification and traceability throughout manufacturing processes.
- Document Review: Familiarity with reviewing and ensuring Good Document Practices (GDP) for Routers, Inspection Plans, and Device History Records (DHRs).
- Quality Systems: Experience using ERP and QMS systems for documenting and controlling inspection and traceability activities.
- Packaging & Documentation: Knowledge of generating Packing Slips and Certificates of Compliance (C of C) according to requirements.
- Records Management: Familiarity with physical and electronic record retention procedures.
Other Skills
- Attention to Detail: Demonstrated ability to work meticulously and accurately.
- Problem-Solving: Skill in identifying and resolving quality issues with machined components.
- Communication: Effective communication skills to interact with colleagues, supervisors, and customers.
- Safety: Commitment to following occupational safety and health requirements, including PPE use.
Additional Notes
- The specific requirements of the role may vary depending on the company and industry.
- Familiarity with the relevant regulations and standards for the industry is a plus.
- Candidates with experience in the manufacturing environment will be at an advantage.
Qualifications:
Education
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
Experience
- Minimum of 3-5 years’ experience in a regulated manufacturing environment.
Qualifications
- Regulated manufacturing industry experience (e.g. Aerospace, Defense, Pharmaceutical, etc.)
- Ability to effectively read and understand blueprints, specifications and procedures.
- Knowledge of dimensional, visual and mechanical inspection processes.
- Strong background concerning the usage and care of mechanical measurement equipment, tools and hand-held tools including micrometers, calipers, dial indicators, height gages, pin gages, etc.
- Maintains accuracy and attention to detail at all times and completes tasks in a timely manner.
- “Hands-on” self-starter with ability to work both independently and as part of a team.
- Strong verbal and written English language communication skills.
- Competency with Microsoft Office (i.e. Outlook, Word, Excel, and PowerPoint).
- Ability to work with a variety of functional areas, including R&D, Manufacturing, and QA, as required, accomplishing results with minimal guidance.
Preferred Skills
- Quality certification(s) (e.g. ASQ CQT, QCI, etc.).
- Class I, II and/or III Medical Device manufacturing experience.
- Knowledge of and experience with GMP/ISO standards.
Physical Demands
While performing the duties of this job, the employee is frequently required to sit, talk and/or hear, and/or use hands to finger, handle, or touch objects, tools, or controls. The employee is occasionally required to stand, and/or walk. The employee must occasionally lift and/or move up to 20 pounds while moving files or small packages. The mental and physical requirements described here are representative of those that must be met by an individual to successfully perform the essential functions of this position.
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Quality Inspector to join our organization. TOMZ offers competitive compensat...
Our Mission
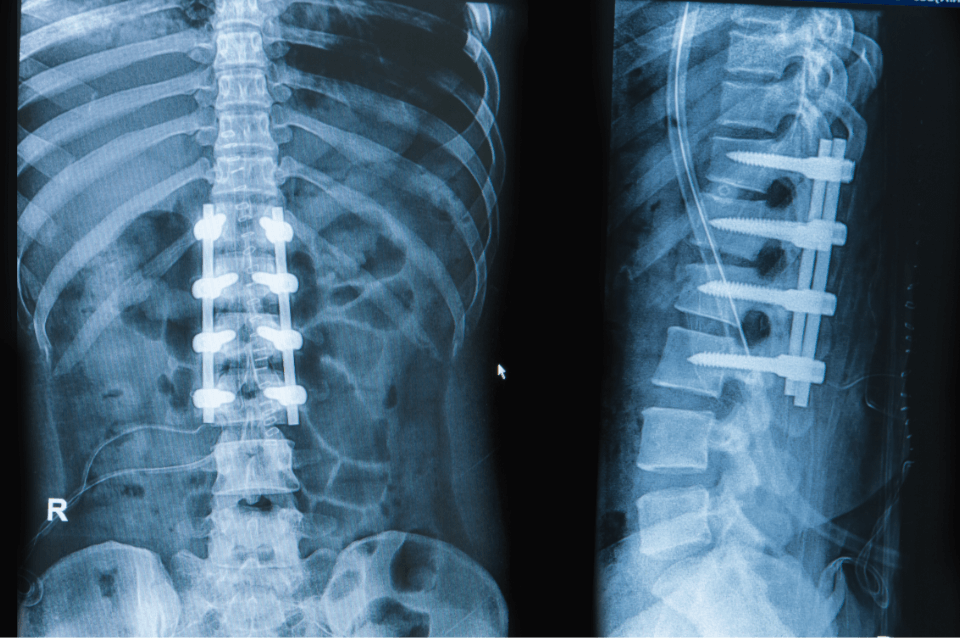
To enhance the lives of patients by sustainably manufacturing innovative medical devices for leading OEMs around the globe.


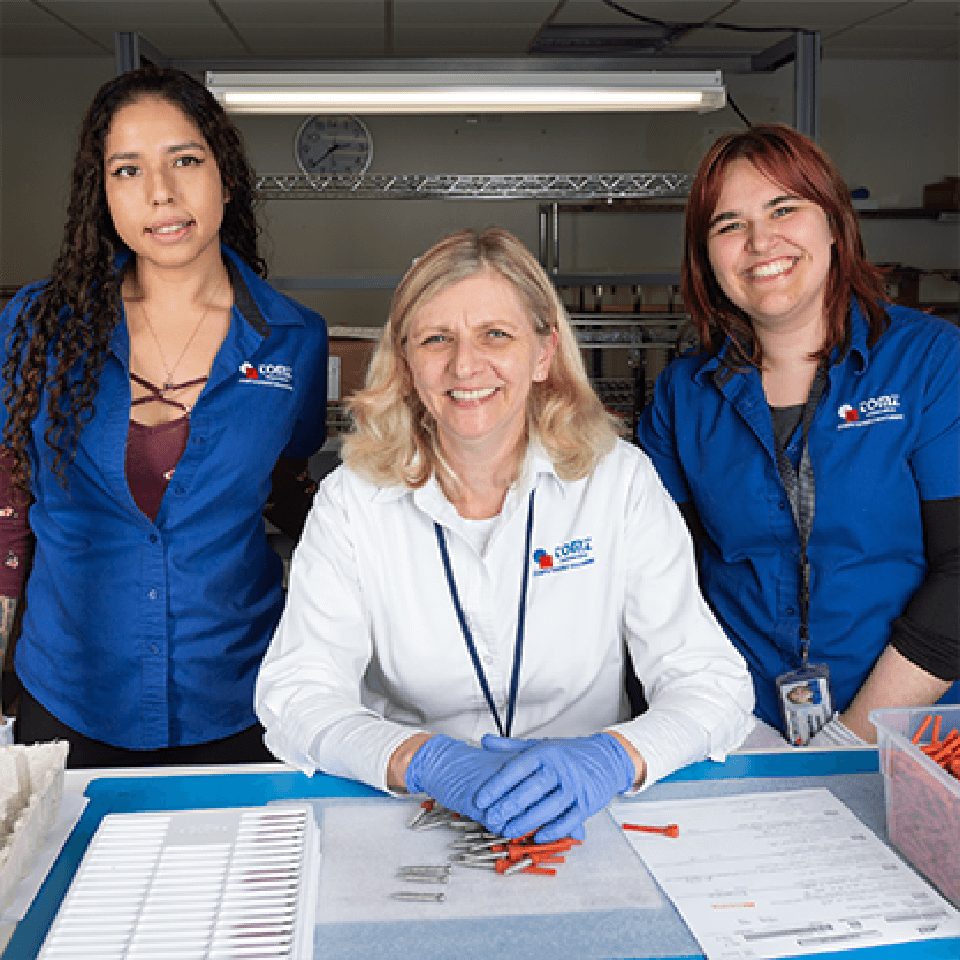
We Manufacture Medical Devices
We specialize in manufacturing the best medical device implants for medical teams and surgeons around the world. Most of our team will tell you that the work is very fulfilling because we help people live better lives.
Company Values & Culture
Our DNA is as unique as everyone that works here.
Put Patients First
Embrace Diversity

Learn & Grow

Reward Great Effort
Why Work at TOMZ?
We are large enough to offer stability and great benefits, yet small enough to recognize you as an individual and not just “another employee.”
Multiple departments from facilities management to engineering allows you to explore different career paths.
The medical devices industry is experiencing explosive growth with no signs of stopping any time soon.
We have completed our 8th expansion which includes amenities such as locker rooms, showers, an expanded cafeteria, and more.
We have a deep bench of knowledgeable pros to help you.
Everything from 401K to top-of-the-line healthcare, life insurance, and more!
What Our Team Members Have to Say
Playlist
Benefits
401(k) Retirement Plan
We will match $0.50 on the dollar up to an 8% contribution. Work with us, retire with us.
Overtime Available
Opportunities to earn overtime hours are available as needed.
Paid Holidays
9 paid holidays AND enjoy a half-day the work day before the paid holiday.
Paid Vacation
Paid vacation starts accruing from the day you start.
Referral Bonus
All employees are eligible to earn a $3,500 referral bonus!
Health Insurance Plans
Enjoy a low employee co-pay with high contributions towards your plan.
Dental Insurance Plans
Yes - we have a great dental plan as well!
Annual Bonus
Production teams achieving set performance goals receive an annual performance bonus.

