

Imagine yourself at the heart of innovation with TOMZ Corporation, where every turn of the lathe leads to groundbreaking results. As an Expert CNC Machinist Technician, you will be embedded in a culture at the forefront of medical device manufacturing. If you’re seeking an opportunity packed with competitive packages, detailed health benefits, and a cutting-edge environment, look no further!
Day in the Life
The hum of precision machines will surround you as you report to the Operations Supervisor, spearheading the production of pivotal components. You'll ensure that our high-precision machining, finishing, assembly, and anodizing techniques meet the strict ISO 13485 standards.
Essential Functions
Duties include but are not limited to:
- Set up and operate Citizen and Tsugami Lathes.
- Implement tool changes and offsets to maintain seamless production.
- Handle setups for new and existing production, guaranteeing efficient machine operation.
- Execute stringent visual and dimensional inspections with finesse using precision instruments.
- Maintain machinery through a regular schedule of preventative actions.
- Standardize quality inspections and documentation via ERP and QMS systems.
- Contribute to safety by observing occupational health standards and PPE requirements.
Qualifications:
Education/Experience
- High school diploma or equivalent; technical school certifications are desirable.
- 2-5 years immersed in a regulated manufacturing setting.
Preferred Skills/Qualifications
- Experience: You've sharpened your skills in Class I, II, or III medical device manufacturing.
- Technical Mastery: Proficient in machine and shop math, you are familiar with GMP/ISO standards.
- Collaborative Spirit: A self-starter who thrives both independently and in integrated teams.
- Detail-Oriented: Recognizing and resolving nonconformances with precision is second nature to you.
- Blueprint Savvy: Comfortable interpreting blueprints and precise in applying GD&T standards.
Physical Demands
- Engage in tasks that require bending, lifting, and precise manual coordination.
- Safely maneuver through noise, particulate exposure, and diverse industrial conditions using PPE.
Become a vital thread in the fabric of TOMZ Corporation, where your expertise and dedication transform the industry every day.
Harness the power of CNC Mill (3-4 axis VMC) and CNC Swiss Lathe technology, and tackle challenges with CNC Lathe (5+ axis) innovation!
Imagine yourself at the heart of innovation with TOMZ Corporation, where every turn of the lathe leads to groundbreaking results. As an Expert CNC Machinist Technician, you will be embedded in a cultu...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and certified to ISO 13485.
Summary of Position
Reporting to the Operations Supervisor, this position will have direct responsibility for the set-up and manufacture of components within the Swiss machining department. TOMZ manufactures parts via high- precision metal machining, finishing, assembly, and anodizing processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, set up and operate equipment, and perform visual and dimensional inspections on machined components using various precision measuring equipment.
Essential Functions
- Operate and maintain Citizen Lathes, Tsugami Lathes, Index multi-spindle lathes, 3-4-5 axis production equipment, including Robo Drills, Mill Turn, and horizontal/vertical mills.
- Perform offsets and tool changes to ensure efficient production with minimal support.
- Set up complex legacy components and new production orders for steady machine operation.
- Conduct visual and dimensional inspections using microscopes, micrometers, calipers, pin gauges, thread gauges, comparators, Micro-Hite, CMM, and vision systems.
- Perform scheduled and regular preventive maintenance on equipment.
- Drive problem-solving activities for complex machine or process issues.
- Complete and compile documentation related to quality inspection standards.
- Ensure proper material control, identification, and traceability for conforming and nonconforming materials.
- Support Quality Best Practices and contribute to continuous improvement in GDP/GMP.
- Capable of mentoring and training lower-level machinists.
- Facilitate a continuous improvement in culture and ensure quality and delivery objectives are met.
- Utilize ERP and QMS systems to document and control inspection and traceability activities.
- Maintain safe working conditions and follow occupational safety and health requirements, including PPE guidance.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Education
- Certification from a technical school program or equivalent, directly transferable work experience in a manufacturing or engineering discipline may be accepted.
- Certification from a technical school program is strongly preferred.
Experience
- Minimum of 3-7 years’ experience in a regulated manufacturing environment.
Qualifications
- Experience in Class I, II, and/or III medical device manufacturing within a regulated environment using calibrated equipment and hazardous materials.
- Relevant job experience in regulated manufacturing, with industry experience in aerospace, defense, or automotive.
- Knowledge of and experience with GMP/ISO standards.
- A self-starter capable of working independently and as part of a team, with the ability to collaborate across various functional areas, including R&D, Manufacturing, and QA, to achieve results with minimal guidance.
- Advanced understanding of mechanical equipment usage, care, and inspection processes.
- Ability to use hand-held tools for preventive maintenance, repairs, and tool changes.
- Skilled in reading and understanding of blueprints, specifications, and procedures.
- Detail-oriented with the ability to maintain accuracy and complete tasks promptly.
- Proficient in identifying nonconformances and maintaining organization.
- Intermediate skills in GD&T.
Preferred Skills
- Strong verbal and written English language communication skills.
- Personal protective equipment (PPE) such as safety glasses, dust masks, ear plugs, cut-resistant or chemical-resistant gloves, and engineering controls may be required.
- Ability to sit or stand for prolonged periods.
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive com...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and certified to ISO 13485.
Summary of Position
Reporting to the Operations Manager, this position will have direct responsibility for the set-up and manufacture of components within the machining Department. TOMZ manufactures parts via high precision metal machining, finishing, assembly, and anodizing processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, set up and operate equipment, and perform visual and dimensional inspections on machine components.
Essential Functions
- Operate and maintain Citizen Lathes, Tsugami Lathes, Index multi-spindle lathes 3-4-5 axis production equipment, including Robo Drills, Mill Turn, and horizontal/vertical mills.
- Perform offsets and tool changes to ensure efficient production.
- Set up complex legacy components and new production orders for steady machine operation.
- Conduct visual and dimensional inspections using microscopes, micrometers, calipers, pin gauges, thread gauges, comparators, Micro-Hite, CMM, vision systems.
- Perform scheduled and regular preventive maintenance on equipment.
- Lead and train in problem solving activities for complex machine or process issues.
- Complete and compile documentation related to quality inspection standards.
- Ensure proper material control, identification, and traceability for conforming and nonconforming materials.
- Lead and train in Quality Best Practices and contribute to continuous improvement in GDP/GMP.
- Utilize ERP and QMS systems to document and control inspection and traceability activities.
- Perform Zeiss (or similar) CMM Gauge R&R activities for NPI.
- Perform all AQL in production with all data being entered into the ERP system.
- Perform Qualification runs on NPI to the customers specifications.
- Perform the first article inspection with minimal support.
- Capable of mentoring and training lower-level machinists.
- Perform FANUC (or similar) robot adjustments on basic programs.
- Maintain safe working conditions and follow occupational safety and health requirements, including PPE guidance.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Education
- Certification from a Machine trades technical school program, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
Experience
- Certification from a Machine trades technical school program and ten years relevant work experience in a regulated medical manufacturing environment, or fifteen years in a regulated medical manufacturing environment.
Qualifications
- Experience in Class I, II, and/or III medical device manufacturing within a regulated environment using calibrated equipment and hazardous materials.
- Knowledge of and experience with GMP/ISO standards.
- Advanced understanding of mechanical equipment usage, care, and inspection processes.
- Ability to use hand-held tools for preventive maintenance, repairs, and tool changes.
- A self-starter capable of working independently and as part of a team, with the ability to collaborate across various functional areas, including R&D, Manufacturing, and QA, to achieve results with minimal guidance.
- Proficient reading and understanding of blueprints, specifications, and procedures.
- Detail-oriented with the ability to maintain accuracy and complete tasks promptly.
- Proficient in identifying nonconformances and maintaining organization.
- Advanced skills in Excel and Microsoft word.
- Strong verbal and written English communication skills.
Physical Demands
- Ability to bend, stoop, squat, kneel, and lift up to 50 pounds. Team lift or mechanical assistance required for objects over 50 pounds.
- Perform repetitive hand and arm movements while lifting up to 20 pounds, including gripping, twisting, and placing components for extended periods, and lifting objects overhead.
- 20/20 vision (assisted or unassisted) required for sorting raw and non-conforming materials. Good dexterity is needed for managing small to medium products.
- Exposure to oil, grease, occupational noise, cleaning solvents, dust, metal particles, sparks, coolant, and sharp-edged materials. Personal protective equipment (PPE) such as safety glasses, dust masks, ear plugs, cut-resistant or chemical-resistant gloves, and engineering controls may be required.
- Ability to sit or stand for prolonged periods.
Job Features
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive com...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and certified to ISO 13485.
Summary of Position
Reporting to the Operations Supervisor, this position will have direct responsibility for the manufacturing of components through various basic functions within the Department. TOMZ manufactures parts via high precision metal machining, finishing, assembly, and anodizing processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, operate equipment, and perform visual and dimensional inspections on machine components.
Essential Functions
- Operate and maintain 3-4-5 axis production equipment, including Robo Drills, Mill Turn, and horizontal/vertical mills. Citizen Lathes, Tsugami Lathes, Index multi-spindle lathes.
- Perform visual and dimensional inspections on manufactured components, as required using a microscope, micrometer, caliper, pin gauges, thread gauges, comparator, Micro-Hite, etc.
- Support problem-solving activities for complex machine or process issues.
- Complete and compile documentation related to quality inspection standards.
- Ensure proper material control, identification, and traceability for conforming and nonconforming materials.
- Support Quality Best Practices and contribute to continuous improvement efforts of GDP/GMP.
- Utilize ERP and QMS systems to document and control inspection and traceability activities.
- Must maintain safe working conditions and follow occupational safety and health requirements including Personal Protective Equipment (PPE) guidance and rules.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Experience
- Minimum of 0-2 years’ experience in a regulated manufacturing environment.
Qualifications
- Basic understanding of the usage and care of mechanical equipment and inspection processes.
- Ability to use hand-held tools for preventive maintenance, repairs, and tool changes.
- Ability to effectively read and understand blueprints, specifications, and procedures.
- Detail-oriented with the ability to maintain accuracy and complete tasks promptly.
- Basic understanding of GD&T.
- Knowledge of GMP/ISO standards.
Preferred Skills
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g., aerospace, defense, pharmaceutical, etc.).
- Strong verbal and written English language communication skills
Physical Demands
- Ability to bend, stoop, squat, kneel, and lift up to 50 pounds. Team lift or mechanical assistance required for objects over 50 pounds.
- Perform repetitive hand and arm movements while lifting up to 20 pounds, including gripping, twisting, and placing components for extended periods, and lifting objects overhead.
- 20/20 vision (assisted or unassisted) required for sorting raw and non-conforming materials. Good dexterity is needed for managing small to medium products.
- Exposure to oil, grease, occupational noise, cleaning solvents, dust, metal particles, sparks, coolant, and sharp-edged materials. Personal protective equipment (PPE) such as safety glasses, dust masks, ear plugs, cut-resistant or chemical-resistant gloves, and engineering controls may be required.
- Ability to sit or stand for prolonged periods.
2ND SHIFT: 15% DIFFERENTIAL 5PM-3:30AM
1st shift- No differential
Pay range: $21.00 - $27.00 / hr
Job Features
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive com...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and certified to ISO 13485.
Summary of Position
Reporting to the Operations Supervisor, this position will have direct responsibility for the manufacturing of components through various basic functions within the Department. TOMZ manufactures parts via high precision metal machining, finishing, assembly, and anodizing processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, operate equipment, and perform visual and dimensional inspections on machine components.
Essential Functions
- Operate and maintain 3-4-5 axis production equipment, including Robo Drills, Mill Turn, and horizontal/vertical mills. Citizen Lathes, Tsugami Lathes, Index multi-spindle lathes.
- Perform visual and dimensional inspections on manufactured components, as required using a microscope, micrometer, caliper, pin gauges, thread gauges, comparator, Micro-Hite, etc.
- Support problem-solving activities for complex machine or process issues.
- Complete and compile documentation related to quality inspection standards.
- Ensure proper material control, identification, and traceability for conforming and nonconforming materials.
- Support Quality Best Practices and contribute to continuous improvement efforts of GDP/GMP.
- Utilize ERP and QMS systems to document and control inspection and traceability activities.
- Must maintain safe working conditions and follow occupational safety and health requirements including Personal Protective Equipment (PPE) guidance and rules.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Experience
- Minimum of 0-2 years’ experience in a regulated manufacturing environment.
Qualifications
- Basic understanding of the usage and care of mechanical equipment and inspection processes.
- Ability to use hand-held tools for preventive maintenance, repairs, and tool changes.
- Ability to effectively read and understand blueprints, specifications, and procedures.
- Detail-oriented with the ability to maintain accuracy and complete tasks promptly.
- Basic understanding of GD&T.
- Knowledge of GMP/ISO standards.
Preferred Skills
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g., aerospace, defense, pharmaceutical, etc.)
- Strong verbal and written English language communication skills.
Physical Demands
- Ability to bend, stoop, squat, kneel, and lift up to 50 pounds. Team lift or mechanical assistance required for objects over 50 pounds.
- Perform repetitive hand and arm movements while lifting up to 20 pounds, including gripping, twisting, and placing components for extended periods, and lifting objects overhead.
- 20/20 vision (assisted or unassisted) required for sorting raw and non-conforming materials. Good dexterity is needed for managing small to medium products.
- Exposure to oil, grease, occupational noise, cleaning solvents, dust, metal particles, sparks, coolant, and sharp-edged materials. Personal protective equipment (PPE) such as safety glasses, dust masks, ear plugs, cut-resistant or chemical-resistant gloves, and engineering controls may be required.
- Ability to sit or stand for prolonged periods.
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive com...
Summary of Position
Reporting to Quality leadership, this position will have direct responsibility for the quality control functions within the Department. TOMZ manufactures parts via high precision metal machining, assembly, and anodize processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation and perform inspections on machined components by utilizing conventional layout inspection techniques and equipment.
Essential Functions
- Demonstrate team leadership, train, and provide guidance to Quality Control personnel.
- Aligns and prioritizes workload. Creates targets and expectations for work performance.
- Coaches and corrects team as required.
- Provides input for performance appraisals to employees.
- Train and perform inspections on machined components and assemblies using visual and layout techniques and equipment, such as microscopes, calipers, micrometers, and gages.
- Create, complete, and compile documentation related to Quality Inspection standards, ensuring proper material control, identification, and traceability throughout the manufacturing processes.
- Ensure conformance to Quality standards by reviewing Device History Records (DHRs) and ensuring they align with the Device Master Record (DMR), and that Good Document Practices (GDP) are maintained for Routers, Inspection Plans, and DHRs during the manufacturing process and final lot release.
- Utilize ERP and QMS systems to document inspection activities, ensure traceability, and maintain proper control of records.
- Support continuous improvement efforts aligned with Quality Best Practices and GDP/GMP standards.
- Execute Incoming, In-process, and Final Inspections as assigned, recording nonconformities, ensuring follow-up actions, and moving materials into MRB if issues remain unaddressed.
- Perform material sorts as needed, ensuring nonconformities are identified and addressed.
- Follow safety and health requirements, including Personal Protective Equipment (PPE) guidance and rules, throughout all tasks.
- Adapt to different quality standards, production lines, and teams, and move between multiple departments and production lines as required with a proactive approach.
- Support other Quality Department functions as needed, ensuring flexibility in role responsibilities.
- Support physical and electronic record retention processes as needed.
- Ability to use more complex measurement equipment (Comparator, Vision System, Basic CMM operation)
- Performs process audits to ensure that processes are being performed in accordance with procedures and standard work.
- Trains others to clarify standards and requirements.
- Train and perform further complex inspections on machined components utilizing methods such as comparators, CMM, vision systems, contracers and automated techniques.
- Provide basic programming assistance for complex inspection and test methods, as required.
- Execute First Piece, FAI Inspection, as assigned.
- Supervises and trains others on sorting activities and inspections.
- Evaluates compliance process controls (i.e. SPC, Precontrol, Tool Life, Process Parameter) and train others on process standards.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Education
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
Experience
- Minimum of 5 years’ experience in a regulated manufacturing environment or equivalent education and experience.
Qualifications
- The ability to effectively read and understand blueprints, specifications and procedures.
- Maintains accuracy and attention to detail at all times and completes tasks in a timely manner.
- Knowledge of dimensional, visual, and mechanical inspection processes.
- Competency with Microsoft Office.
- Ability to work both independently and as part of a team.
- Verbal and written English language communication skills.
- Basic mathematical computational ability
- Strong background concerning the usage and care of mechanical measurement equipment, tools and hand-held tools including micrometers, calipers, dial indicators, height gages, pin gages, etc.
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g., Aerospace, Defense, Pharmaceutical, etc.)
Preferred Skills
- Quality certification(s) (e.g., ASQ CQT, QCI, etc.).
- Knowledge of and experience with GMP/ISO standards.
$27- $32 an hour
TOMZ is an Equal Opportunity Employer
Summary of Position Reporting to Quality leadership, this position will have direct responsibility for the quality control functions within the Department. TOMZ manufactures parts via high precision m...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Senior Maintenance Machine Specialist to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
The purpose of the Senior Maintenance Machine Specialist is to provide production equipment support for all equipment allocated to the Factory as well as to assist engineering with production-related projects. Routinely works with Plant Maintenance to coordinate jobs and projects. The Senior Maintenance Machine Specialist will take the lead in the implementation and the decision-making process on best practices for the maintenance group. The position is also responsible for creating solutions and providing technical support to enhance production reliability through concerted efforts with cross functional teams.
Essential Functions
Duties and responsibilities include, but are not limited to:
- Adherence to all department safety measures and work instructions.
- Accountable for personal safety, the safety of co-workers and promoting a safe work environment.
- Repair and maintain all production equipment, both electrical and mechanical.
- Assist engineering as required with special machine related projects.
- Work with CONC and PLC controlled machines.
- Routinely work in electrical control panels of production equipment to troubleshoot electrical problems.
- Arc Flash certified worker to work “live” electrical control panels.
- Working understanding of precision measuring instruments used in troubleshooting.
- Read and interpret diagrams, schematics and specifications related to equipment operation.
- Monitor, modify, or write programmable ladder logic circuits as needed for existing or new installations.
- Create electrical drawings, PLC programs to be used for troubleshooting of modified equipment.
- Install electrical, air and data drops to equipment and machines.
- Work with outside contractors for equipment repair of production machines as needed and verify completion accuracy.
- Design and implement electrical circuits and technologies for support of production equipment.
- Initiate or enhance the preventative maintenance schedules for production equipment.
- Calibrate equipment using general and special purpose test equipment.
- Facilitate equipment modifications, upgrades, overhauls, and root cause failure analyses.
- Ensure respective area is complaint with 5S Standards.
- Maintain daily log of work in established systems.
- Support ISO 13485 initiatives.
- Assist in training individuals with less experience and tenure on established maintenance standards and practices.
- Project initiation and improvement ideas to better production and the business.
- Purchase repair parts, tools, and supplies in appropriate ERP software.
- Provide input for annual department budget planning.
- Ability to work flexible hours (including overtime) to accommodate production working hours as needed.
- Other duties and responsibilities as assigned.
Qualifications:
Education
- Associate degree in a technical related field or equivalent experience
Experience
- Minimum 10 years’ experience in the Equipment Maintenance Field
Qualifications
- “Hands-on” self-starter with ability to work both independently and as part of a team.
- Maintains accuracy, is detail oriented, and completes tasks in a timely manner.
- Prior experience with CNC and PLC controlled equipment.
- Experience with MS Office Programs.
- Ability to perform routine maintenance of CNC manufacturing equipment; troubleshoot issues and support, or directly resolve as appropriate.
- Ability to read and interpret documents and drawings (e.g., blueprints, manuals, wiring diagrams etc.)
- Time management skills and ability to prioritize workflow throughout the day.
- Strong verbal and written English language communication skills.
Preferred Skills
- Forklift and OSHA Training.
Physical Demands
While performing the duties of this job, the employee is frequently required to sit, talk and/or hear, and/or use hands to finger, handle, or touch objects, tools, or controls. The employee is frequently required to stand, and/or walk. The employee must occasionally lift and/or move up to 50 pounds while moving tooling, fixtures, equipment and/or small packages. The mental and physical requirements described here are representative of those that must be met by an individual to successfully perform the essential functions of this position.
Pay Range: $29.81/hr - $43.75/hr
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Senior Maintenance Machine Specialist to join our organization. TOMZ offers c...
Summary of Position
Supervises production team in assigned department(s). Improve safety, quality, delivery, productivity, and cost. Increase team skills and capabilities. Continuously improves associate satisfaction while increasing efficiency.
Essential Functions
- Safe operation of all production processes. Identifies needed improvements and leads the project's implementation. Continually monitors the area and solicits feedback from associates. Enforce all safety rules and regulations.
- Is the key coordinator/communicator for at least one safety training program for the entire organization.
- Supervises team members. Ensures effective employee relations. Resolves issues through problem resolution. Confers with management to resolve complex associate matters.
- Interviews, selects, orients, trains, conducts performance appraisals, performance development, schedules, and approves hours.
- Provides leadership for associate relations through effective communication, coaching, training, and development.
- Executes operational plan to enhance profitability, productivity, and efficiency throughout assigned production department(s).
- Implements Lean Manufacturing techniques to improve quality, cost, on-time delivery, safety, customer satisfaction, and associate satisfaction.
- Maintains production plan.
- Ensures compliance with the organization's standards for cost control, waste reduction, quality, safety, and on-time delivery.
- Provides training and support to the team, sets goals and objectives.
- Measures team performance for safety, quality, delivery, and cost. Work with teams to continually improve these measures.
- Monitors the budget.
- Arranges for equipment, materials, and supplies to be available for work assignments.
- Approves setups and assigns operators. Troubleshoot bottleneck operations to identify the cause and notify the appropriate support team for assistance.
- Checks work methods and operations to make recommendations for improving quality and implement systems to report and reduce scrap and cost.
- Oversees the use of SPC.
- Identifies opportunities aimed at continuous improvement of the quality of products and processes. Leads project team during identification through implementation and evaluation.
- Confers with others to coordinate operations and activities across all shifts.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Education
- Bachelor’s degree or 4-year equivalent directly transferable industry work experience.
Experience
- Minimum of 5 years of manufacturing or engineering experience in a supervisory role.
Qualifications
- Ability to maintain a safe work environment.
- Experience in the setup and operation of manufacturing equipment.
- Knowledge of production and processing and other techniques for maximizing the effective manufacture of our products.
- Broad experience with precision components and assembly.
- Kaizen and Lean Manufacturing techniques.
- High attention to detail and ability to think ahead.
- Knowledge of production cycle.
- Demonstrated ability to lead people and get results through others.
- Solid analytical skills
- Excellent interpersonal and communication skills to work with associates, customers and vendors.
- Excellent project and program management skills.
- Ability to multitask in a fast-paced environment and manage multiple priorities.
- Ability to solve practical problems and deal with a variety of changing situations.
- Quality orientation
- Knowledge of human resources and safety includes coaching, performance management, OSHA and related safety standards.
- Knowledge of mathematics to include geometry and statistics.
- Ability to think critically and use judgment to identify strengths and weaknesses of solutions to problems presented in production.
- Computer skills include word processing, spreadsheets, and databases.
- A self-starter capable of working independently and as part of a team
Physical Demands
- Ability to bend, stoop, squat, kneel, and lift up to 50 pounds. Team lift or mechanical assistance required for objects over 50 pounds.
- Ability to sit or stand for prolonged periods.
- Perform keyboard entries with frequent operation of a computer.
- Exposure to oil, grease, dust, metal particles, sparks, sharp-edged materials. Some exposures may require the use of personal protective equipment or engineering controls.
2nd shift- Start time 2PM
Job Features
Summary of Position Supervises production team in assigned department(s). Improve safety, quality, delivery, productivity, and cost. Increase team skills and capabilities. Continuously improves associ...
Our Mission
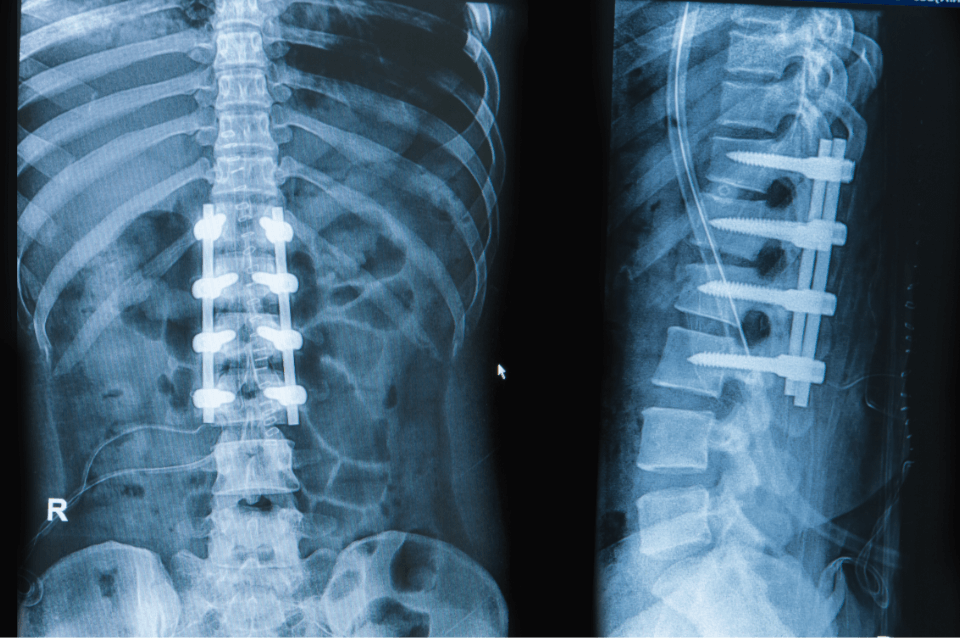
Advancing quality of life by manufacturing medical devices for global healthcare companies.


We Manufacture Medical Devices
We specialize in manufacturing the best medical device implants for medical teams and surgeons around the world. Most of our team will tell you that the work is very fulfilling because we help people live better lives.
Company Values & Culture
Our DNA is as unique as everyone that works here.
Put Patients First
Embrace Diversity

Learn & Grow

Reward Great Effort
Why Work at TOMZ?
We are large enough to offer stability and great benefits, yet small enough to recognize you as an individual and not just “another employee.”
Multiple departments from facilities management to engineering allows you to explore different career paths.
The medical devices industry is experiencing explosive growth with no signs of stopping any time soon.
We have completed our 8th expansion which includes amenities such as locker rooms, showers, an expanded cafeteria, and more.
We have a deep bench of knowledgeable pros to help you.
Everything from 401K to top-of-the-line healthcare, life insurance, and more!
What Our Team Members Have to Say
Playlist
2:37

2:12
Benefits
401(k) Retirement Plan
We will match $0.50 on the dollar up to an 8% contribution. Work with us, retire with us.
Overtime Available
Opportunities to earn overtime hours are available as needed.
Paid Holidays
Enjoy 9 paid holidays off per year.
Paid Time Off (PTO)
PTO starts accruing from the day you start.
Referral Bonus
Employees are eligible to earn a $3,500 referral bonus!
Health Insurance Plans
Enjoy a low employee co-pay with high contributions towards your plan.
Dental Insurance Plans
Yes - we have a great dental plan as well!
Quarterly Bonus
Teams achieving set performance goals receive a quarterly performance bonus.