
Summary of Position
Reporting to Department Manager, this position will have direct responsibility for the setup and manufacture of components within a machining Department. TOMZ manufactures parts via high precision metal machining, finishing, assembly, and anodize processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, setup and operate equipment, and perform visual and dimensional inspections on machined components.
Essential Functions
- Ability to operate and maintain production on equipment within the department. Ex. include Citizen Lathes, Tsugami Lathes, Index multi-spindle lathes, Willemin Mill turns, various 3-4-5 axis mills.
- Demonstrated ability to perform offsets and change tools to maintain efficient production with minimal support.
- Ability to efficiently setup complex legacy components and basic new production orders and ensure the machine is ready for steady production.
- Perform visual and dimensional inspections on manufactured components, as required using microscope, micrometer, caliper, pin gages, thread gages, comparator, micro-hite, CMM, Vision system, etc.
- Regularly and as prescribed, perform preventive maintenance of equipment.
- Drive problem solving activities for complex machine or process issues.
- Completes and compiles necessary documentation related to Quality Inspection standards.
- Ensure proper material control, identification and traceability is maintained for conforming and nonconforming material through the manufacturing processes.
- Support Quality Best Practices and GDP/GMP continuous improvement efforts.
- Utilize and navigate ERP and QMS systems to ensure inspection and traceability activities are properly documented and controlled, as needed.
- Must maintain safe working conditions and follow occupational Safety and Health requirements including Personal Protective Equipment (PPE) guidance and rules.
- Must be knowledgeable of, and adhere to, the TOMZ Quality Management System.
- Other duties and responsibilities are assigned
Qualifications:
Education
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
- Certification from a technical school program strongly preferred.
Experience
- Minimum of 3-7 years’ experience in a regulated manufacturing environment.
Qualifications
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g. Aerospace, Defense, Pharmaceutical, etc.)
- Knowledge of and experience with GMP/ISO standards.
- Advanced understanding of the usage and care of mechanical equipment and inspection processes.
- Ability to use hand-held tools to perform preventive maintenance or repair activities and install or change tools.
- “Hands-on” self-starter with ability to work both independently and as part of a team.
- Ability to effectively read and understand blueprints, specifications, and procedures.
- Maintains accuracy and attention to detail at all times and completes tasks in a timely manner.
- Ability to work with a variety of functional areas, including R&D, Manufacturing, and QA, as required, accomplishing results with minimal guidance.
Preferred Skills
- Strong verbal and written English language communication skills.
Physical Demands
- Occasionally lift/move up to 50 pounds this may be performed with assistants if needed.
- Prolonged periods of sitting.
Pay Range: $26/hr - $36/hr
Summary of Position Reporting to Department Manager, this position will have direct responsibility for the setup and manufacture of components within a machining Department. TOMZ manufactures parts vi...
Summary of Position
Reporting to the Operations Supervisor, this position will have direct responsibility for the set-up and manufacture of components within the Swiss machining department. TOMZ manufactures parts via high- precision metal machining, finishing, assembly, and anodizing processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, set up and operate equipment, and perform visual and dimensional inspections on machined components using various precision measuring equipment.
Essential Functions
- Operate and maintain Citizen Lathes, Tsugami Lathes, Index multi-spindle lathes, 3-4-5 axis production equipment, including Robo Drills, Mill Turn, and horizontal/vertical mills.
- Perform offsets and tool changes to ensure efficient production with minimal support.
- Set up complex legacy components and new production orders for steady machine operation.
- Conduct visual and dimensional inspections using microscopes, micrometers, calipers, pin gauges, thread gauges, comparators, Micro-Hite, CMM, and vision systems.
- Perform scheduled and regular preventive maintenance on equipment.
- Drive problem-solving activities for complex machine or process issues.
- Complete and compile documentation related to quality inspection standards.
- Ensure proper material control, identification, and traceability for conforming and nonconforming materials.
- Support Quality Best Practices and contribute to continuous improvement in GDP/GMP.
- Capable of mentoring and training lower-level machinists.
- Facilitate a continuous improvement in culture and ensure quality and delivery objectives are met.
- Utilize ERP and QMS systems to document and control inspection and traceability activities.
- Maintain safe working conditions and follow occupational safety and health requirements, including PPE guidance.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Education
- Certification from a technical school program or equivalent, directly transferable work experience in a manufacturing or engineering discipline may be accepted.
- Certification from a technical school program is strongly preferred.
Experience
- Minimum of 3-7 years’ experience in a regulated manufacturing environment.
Qualifications
- Experience in Class I, II, and/or III medical device manufacturing within a regulated environment using calibrated equipment and hazardous materials.
- Relevant job experience in regulated manufacturing, with industry experience in aerospace, defense, or automotive.
- Knowledge of and experience with GMP/ISO standards.
- A self-starter capable of working independently and as part of a team, with the ability to collaborate across various functional areas, including R&D, Manufacturing, and QA, to achieve results with minimal guidance.
- Advanced understanding of mechanical equipment usage, care, and inspection processes.
- Ability to use hand-held tools for preventive maintenance, repairs, and tool changes.
- Skilled in reading and understanding of blueprints, specifications, and procedures.
- Detail-oriented with the ability to maintain accuracy and complete tasks promptly.
- Proficient in identifying nonconformances and maintaining organization.
- Intermediate skills in GD&T.
Preferred Skills
- Strong verbal and written English language communication skills.
Physical Demands
- Ability to bend, stoop, squat, kneel, and lift up to 50 pounds. Team lift or mechanical assistance required for objects over 50 pounds.
- Perform repetitive hand and arm movements while lifting up to 20 pounds, including gripping, twisting, and placing components for extended periods, and lifting objects overhead.
- 20/20 vision (assisted or unassisted) required for sorting raw and non-conforming materials. Good dexterity is needed for managing small to medium products.
- Exposure to oil, grease, occupational noise, cleaning solvents, dust, metal particles, sparks, coolant, and sharp-edged materials. Personal protective equipment (PPE) such as safety glasses, dust masks, ear plugs, cut-resistant or chemical-resistant gloves, and engineering controls may be required.
- Ability to sit or stand for prolonged periods.
WEEKEND SHIFT: 20% DIFFERENTIAL 5AM-5PM FRI-SUN
2ND SHIFT: 15% DIFFERENTIAL 5PM-3:30AM
1st shift - No differential
Pay Range: $31.00 - $37.00
Job Features
Summary of Position Reporting to the Operations Supervisor, this position will have direct responsibility for the set-up and manufacture of components within the Swiss machining department. TOMZ manuf...
Summary of Position
Reporting to the Operations Supervisor, this position will have direct responsibility for the manufacturing of components through various basic functions within the Department. TOMZ manufactures parts via high precision metal machining, finishing, assembly, and anodizing processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, operate equipment, and perform visual and dimensional inspections on machine components.
Essential Functions
- Operate and maintain 3-4-5 axis production equipment, including Robo Drills, Mill Turn, and horizontal/vertical mills. Citizen Lathes, Tsugami Lathes, Index multi-spindle lathes.
- Perform visual and dimensional inspections on manufactured components, as required using a microscope, micrometer, caliper, pin gauges, thread gauges, comparator, Micro-Hite, etc.
- Support problem-solving activities for complex machine or process issues.
- Complete and compile documentation related to quality inspection standards.
- Ensure proper material control, identification, and traceability for conforming and nonconforming materials.
- Support Quality Best Practices and contribute to continuous improvement efforts of GDP/GMP.
- Utilize ERP and QMS systems to document and control inspection and traceability activities.
- Must maintain safe working conditions and follow occupational safety and health requirements including Personal Protective Equipment (PPE) guidance and rules.
- Adhere to the TOMZ Quality Management System.
- Perform other duties as assigned.
Qualifications:
Experience
- Minimum of 0-2 years’ experience in a regulated manufacturing environment.
Qualifications
- Basic understanding of the usage and care of mechanical equipment and inspection processes.
- Ability to use hand-held tools for preventive maintenance, repairs, and tool changes.
- Ability to effectively read and understand blueprints, specifications, and procedures.
- Detail-oriented with the ability to maintain accuracy and complete tasks promptly.
- Basic understanding of GD&T.
- Knowledge of GMP/ISO standards.
Preferred Skills
- Class I, II and/or III Medical Device manufacturing experience.
- Regulated manufacturing industry experience (e.g., aerospace, defense, pharmaceutical, etc.)
- Strong verbal and written English language communication skills.
Physical Demands
- Ability to bend, stoop, squat, kneel, and lift up to 50 pounds. Team lift or mechanical assistance required for objects over 50 pounds.
- Perform repetitive hand and arm movements while lifting up to 20 pounds, including gripping, twisting, and placing components for extended periods, and lifting objects overhead.
- 20/20 vision (assisted or unassisted) required for sorting raw and non-conforming materials. Good dexterity is needed for managing small to medium products.
- Exposure to oil, grease, occupational noise, cleaning solvents, dust, metal particles, sparks, coolant, and sharp-edged materials. Personal protective equipment (PPE) such as safety glasses, dust masks, ear plugs, cut-resistant or chemical-resistant gloves, and engineering controls may be required.
- Ability to sit or stand for prolonged periods.
Summary of Position Reporting to the Operations Supervisor, this position will have direct responsibility for the manufacturing of components through various basic functions within the Department. TOM...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and certified to ISO 13485.
Summary of Position
Reporting to the Operations Supervisor, this position will have direct responsibility for the set-up and manufacture of components within a machining Department. TOMZ manufactures parts via high- precision metal machining, finishing, assembly, and anodizing processes for Medical Devices. The successful candidate will be expected to maintain the ISO 13485 standards documentation, set up and operate equipment, and perform visual and dimensional inspections on machined components using various precision measuring equipment.
Essential Functions
- Set up and operate and maintain Citizen Lathes or Tsugami Lathes
- Perform offsets and tool changes to ensure efficient production with minimal support.
- Set up legacy components and new production orders for steady machine operation.
- Perform preventative maintenance on Swiss Lathe to ensure consistent machine performance
- Conduct visual and dimensional inspections using microscopes, micrometers, calipers, pin gauges, thread gauges, comparators, Micro-Hite, CMM, and vision systems.
- Perform scheduled and regular preventive maintenance on equipment.
- Drive problem-solving activities for complex machine or process issues.
- Complete and compile documentation related to quality inspection standards.
- Ensure proper material control, identification, and traceability for conforming and nonconforming materials.
- Support Quality Best Practices and contribute to continuous improvement in GDP/GMP.
- Utilize ERP and QMS systems to document and control inspection and traceability activities.
- Maintain safe working conditions and follow occupational safety and health requirements, including PPE guidance.
Qualifications:
Education
- High school diploma, GED, or equivalent directly transferrable work experience (Manufacturing or Engineering discipline preferred).
- Certification from a technical school program preferred.
Experience
- Minimum of 2-5 years’ experience in a regulated manufacturing environment.
Qualifications
- Experience in Class I, II, and/or III medical device manufacturing within a regulated environment using calibrated equipment and hazardous materials.
- Strong math background, preferable in machine math/shop math
- Relevant job experience in regulated manufacturing, with industry experience in aerospace, defense, or automotive.
- Knowledge of and experience with GMP/ISO standards.
- A self-starter capable of working independently and as part of a team, with the ability to collaborate across various functional areas, including R&D, Manufacturing, and QA, to achieve results with guidance.
- Understanding of mechanical equipment usage, care, and inspection processes.
- Ability to use hand-held tools for preventive maintenance, repairs, and tool changes.
- Effectively read and understand blueprints, specifications and procedures.
- Understanding of GD&T.
- Detail-oriented with the ability to maintain accuracy and complete tasks promptly.
- Identify nonconformances and maintain organization.
Preferred Skills
- Strong verbal and written English language communication skills.
Physical Demands
- Ability to bend, stoop, squat, kneel, and lift up to 50 pounds. Team lift or mechanical assistance required for objects over 50 pounds.
- Perform repetitive hand and arm movements while lifting up to 20 pounds, including gripping, twisting, and placing components for extended periods, and lifting objects overhead.
- 20/20 vision (assisted or unassisted) required for sorting raw and non-conforming materials. Good dexterity is needed for managing small to medium products.
- Exposure to oil, grease, occupational noise, cleaning solvents, dust, metal particles, sparks, coolant, and sharp-edged materials. Personal protective equipment (PPE) such as safety glasses, dust masks, ear plugs, cut-resistant or chemical-resistant gloves, and engineering controls may be required.
- Ability to sit or stand for prolonged periods
1st shift: 6AM-2:30PM
2nd shift: 5PM-1:30AM (15% SHIFT DIFFERENTIAL)
Weekend shift: 6AM-6PM (20% SHIFT DIFFERENTIAL)
Job Features
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a motivated CNC Machinist to join our organization. TOMZ offers competitive com...
Join our team in Fishers, Indiana! TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a highly motivated Staff Quality Engineer to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and certified to ISO 13485.
Summary of Position
This position will be responsible for the activities associated with Quality Engineering, Control and Assurance continuation development support for continuous improvement of manufacturing processes.
Essential Functions
- Author and execute Manufacturing Quality Plans, Control Plans and Master Validation Plans.
- Leads on strategic initiatives to improve Quality Processes and Systems.
- Owns the development and/or update of Risk Management files in cooperation with cross-functional engineering teams, operations and customers.
- Supports the procurement and qualification of capital equipment.
- Operates and programs advanced measurement equipment including Vision Systems and CMMs.
- Authors Inspection Plans and defines process controls. Defines Test Method.
- Subject Matter expertise of precision measuring instruments and their application, including but not limited to, calipers, micrometers, depth gages, indicators, and plug and thread Go/No Go gages.
- Execution and evaluation of Test Method Validations.
- Creation of production visual standards and standardized acceptance criteria for TOMZ’s manufacturing processes.
- Initiate and/or consult in the development and/or update of Risk Management documentation in cooperation with cross-functional engineering teams per project requirements.
- Performs process qualifications, analyzes data and initiates corrections or corrective actions as required
- Support and enforce Quality Best Practices and GDP/GMP continuous improvement efforts.
- Apply sound, systematic problem-solving methodologies in identifying, prioritizing, communicating, resolving quality issues through the CAPA process.
- Defines and Measures KPI’s for New Product Introduction.
- Interfaces with the customer and meets all defined deliverables.
- Support the tracking and reporting of inspection data, including but not limited to rejection rates, defect modes and process trends across customers/product families.
- Generate ECOs for the release of Quality Engineering documentation.
Qualifications:
Education/Experience
- Bachelor's or higher degree in Engineering, Manufacturing, or Business discipline or equivalent of directly transferable industry work experience.
- Minimum of 0-4 years’ experience in a regulated manufacturing environment.
- Engineering degree and 7+ years of manufacturing quality experience including advanced metrology experience including CMM and/or Vision System programming. (12+ years of experience in Quality Engineering or similar discipline will be considered in lieu of a degree)
Qualifications
- Ability to travel up to 20%.
- Demonstrated self-starter with ability to work in a fast-paced environment.
- Competency with Microsoft Office (Visio, Project, Outlook, Word, Excel, and PowerPoint)
- Strong verbal and technical writing capabilities (English language).
- Expertise is statistical techniques, GD&T, process validation, and Metrology.
- Demonstrated ability to work in and lead teams through large scale projects.
Preferred Skills
- ASQ CQE or CRE.
- Expertise in 3D modeling Solid Works, Unigraphics, Mastercam, Solid Edge, etc.
- Six Sigma Green or Black belt.
- Certifications in DMIS programming preferred
- Regulated manufacturing industry experience preferably Class I, II and/or III Medical Device manufacturing experience. (exp: Aerospace, Defense, Pharmaceutical, etc.)
- Knowledge and experience with external standards: ISO 9000/9001, ISO 13485, and 21CFR 820, EU MDR, especially pertaining to product development, design controls, good manufacturing practices, supplier qualification, auditing, quality control (GD&T, Nonconforming Materials, MRB), Corrective and Preventive Actions, and customer complaints).
Join our team in Fishers, Indiana! TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a highly motivated Staff Quality Engineer to...
Summary of Position
Join our team in Berlin, CT!! Reporting to the Engineering Manager, the Manufacturing Engineer will have direct responsibility for developing and implementing robust cost-effective manufacturing processes and methods, recommends and improvements to sustained production processes, methods, and controls, as well as coordinate the launch of new products into volume production. TOMZ manufactures parts via high precision metal machining, finishing, assembly, and anodize processes for Medical Devices.
Essential Functions
- Support Production in reducing / eliminating equipment and tooling downtime or scrap.
- Perform tool life studies to drive efficiency and cost savings opportunities.
- Conduct Process Capability studies during manufacturing processes to support achieving objectives.
- Collaborates with cross-functional teams during the production transfer phase to establish and launch robust, mid to high volume processes.
- Improves manufacturing processes, methods and controls for cost reduction, quality improvements and efficiency for both sustained and new products.
- Prepares engineering change orders and coordinates the deployment of changes including training operation team members.
- Develop and improve manufacturing process instructions, product flow, assembly methods, space allocation, product quality and safety performance for both sustained and new products.
- Additional responsibilities as outlined in the full job description.
Qualifications:
Education
- Minimum of a bachelor’s degree in an engineering discipline or equivalent experience.
Experience
- Minimum of 5 years’ experience within a regulated industry (preferably in a Medical Device or Aerospace contract Manufacturer).
Qualifications
- Must possess a high attention to detail.
- Ability to read drawings and specifications containing GD&T.
- Proficiency in SolidWorks.
- Proficient in the use of Microsoft Office and Adobe products.
- Solid knowledge of GMP and ISO 13485 regulations.
- Experience with continuous improvement activities, including participation and/or facilitating Kaizen events using lean manufacturing principles.
- Ability to work in a fast-paced Medical Device Manufacturing environment with high output efficiency and impeccable quality standards.
TOMZ Corporation is an Equal Opportunity Employer
Summary of Position Join our team in Berlin, CT!! Reporting to the Engineering Manager, the Manufacturing Engineer will have direct responsibility for developing and implementing robust cost-effective...
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Senior Machine Maintenance Specialist to join our organization. TOMZ offers competitive compensation and excellent benefits, including 401k, health/dental, vision, and paid time off. TOMZ maintains a modern, clean, and safe facility with state-of-the-art equipment and dedication to continuous process improvement. TOMZ is registered with the FDA as a Class 2 and Class 3 Medical Device Manufacturer and is certified to ISO 13485.
Summary of Position
The purpose of the Senior Machine Maintenance Specialist is to provide production equipment support for all equipment allocated to the Factory as well as to assist engineering with production-related projects. Routinely works with Plant Maintenance to coordinate jobs and projects. The Senior Machine Specialist will take the lead in the implementation and the decision-making process on best practices for the maintenance group. The position is also responsible for creating solutions and providing technical support to enhance production reliability through concerted efforts with cross functional teams.
Essential Functions
Duties and responsibilities include, but are not limited to:
- Adherence to all department safety measures and work instructions.
- Accountable for personal safety, the safety of co-workers and promoting a safe work environment.
- Repair and maintain all production equipment, both electrical and mechanical.
- Assist engineering as required with special machine related projects.
- Work with CONC and PLC controlled machines.
- Routinely work in electrical control panels of production equipment to troubleshoot electrical problems.
- Arc Flash certified worker to work “live” electrical control panels.
- Working understanding of precision measuring instruments used in troubleshooting.
- Read and interpret diagrams, schematics and specifications related to equipment operation.
- Monitor, modify, or write programmable ladder logic circuits as needed for existing or new installations.
- Create electrical drawings, PLC programs to be used for troubleshooting of modified equipment.
- Install electrical, air and data drops to equipment and machines.
- Work with outside contractors for equipment repair of production machines as needed and verify completion accuracy.
- Design and implement electrical circuits and technologies for support of production equipment.
- Initiate or enhance the preventative maintenance schedules for production equipment.
- Calibrate equipment using general and special purpose test equipment.
- Facilitate equipment modifications, upgrades, overhauls, and root cause failure analyses.
- Ensure respective area is complaint with 5S Standards.
- Maintain daily log of work in established systems.
- Support ISO 13485 initiatives.
- Assist in training individuals with less experience and tenure on established maintenance standards and practices.
- Project initiation and improvement ideas to better production and the business.
- Purchase repair parts, tools, and supplies in appropriate ERP software.
- Provide input for annual department budget planning.
- Ability to work flexible hours (including overtime) to accommodate production working hours as needed.
- Other duties and responsibilities as assigned.
Qualifications:
Education
- Associate degree in a technical related field or equivalent experience
Experience
- Minimum 10 years’ experience in the Equipment Maintenance Field
Qualifications
- “Hands-on” self-starter with ability to work both independently and as part of a team.
- Maintains accuracy, is detail oriented, and completes tasks in a timely manner.
- Prior experience with CNC and PLC controlled equipment.
- Experience with MS Office Programs.
- Ability to perform routine maintenance of CNC manufacturing equipment; troubleshoot issues and support, or directly resolve as appropriate.
- Ability to read and interpret documents and drawings (e.g., blueprints, manuals, wiring diagrams etc.)
- Time management skills and ability to prioritize workflow throughout the day.
- Strong verbal and written English language communication skills.
Preferred Skills
- Forklift and OSHA Training.
Physical Demands
While performing the duties of this job, the employee is frequently required to sit, talk and/or hear, and/or use hands to finger, handle, or touch objects, tools, or controls. The employee is frequently required to stand, and/or walk. The employee must occasionally lift and/or move up to 50 pounds while moving tooling, fixtures, equipment and/or small packages. The mental and physical requirements described here are representative of those that must be met by an individual to successfully perform the essential functions of this position.
TOMZ Corporation, a leader in manufacturing of devices and components for major medical device companies, is looking for a Senior Machine Maintenance Specialist to join our organization. TOMZ offers c...
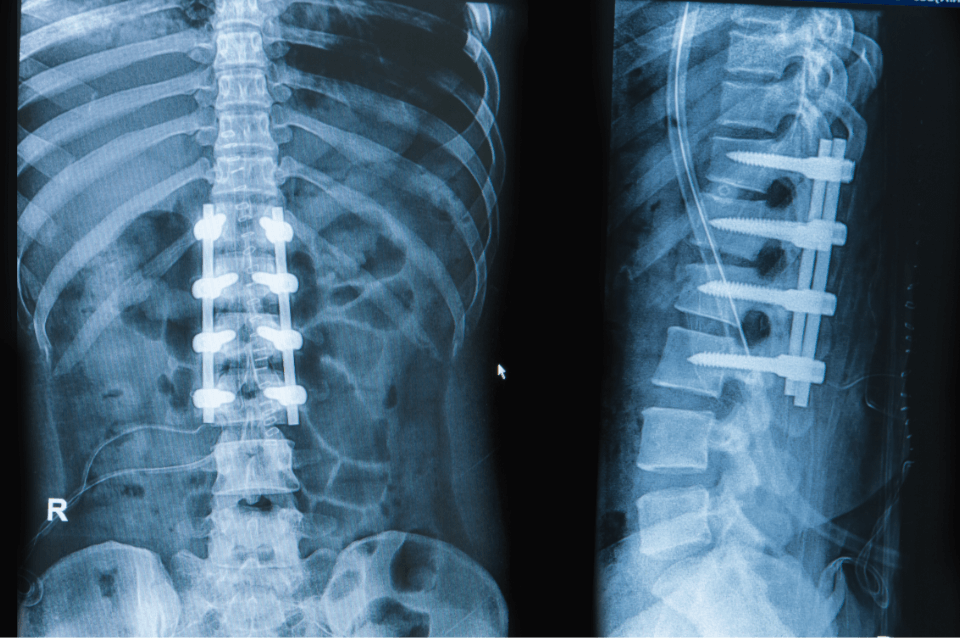
Our Mission
Advancing quality of life by manufacturing medical devices for global healthcare companies.


We Manufacture Medical Devices
We specialize in manufacturing the best medical device implants for medical teams and surgeons around the world. Most of our team will tell you that the work is very fulfilling because we help people live better lives.
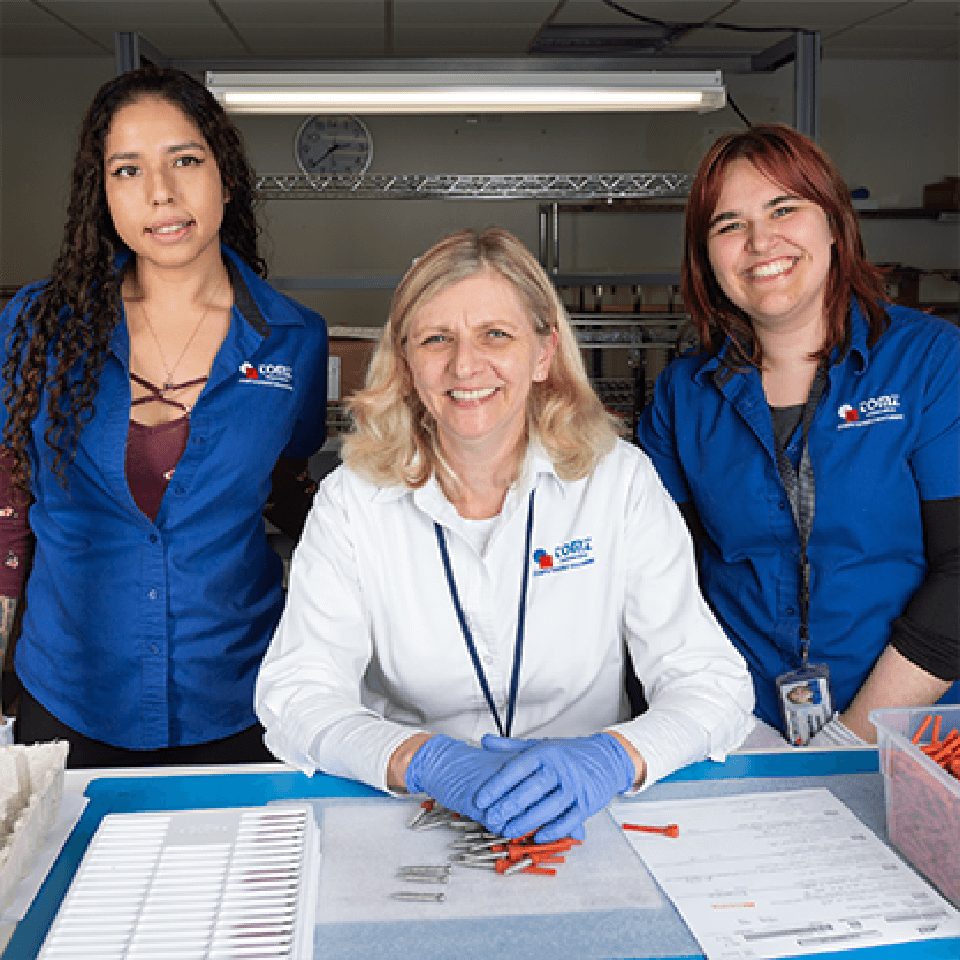
Company Values & Culture
Our DNA is as unique as everyone that works here.
Put Patients First
Embrace Diversity

Learn & Grow

Reward Great Effort
Why Work at TOMZ?
We are large enough to offer stability and great benefits, yet small enough to recognize you as an individual and not just “another employee.”
Multiple departments from facilities management to engineering allows you to explore different career paths.
The medical devices industry is experiencing explosive growth with no signs of stopping any time soon.
We have completed our 8th expansion which includes amenities such as locker rooms, showers, an expanded cafeteria, and more.
We have a deep bench of knowledgeable pros to help you.
Everything from 401K to top-of-the-line healthcare, life insurance, and more!
What Our Team Members Have to Say
Playlist
2:37

2:12
Benefits
401(k) Retirement Plan
We will match $0.50 on the dollar up to an 8% contribution. Work with us, retire with us.
Overtime Available
Opportunities to earn overtime hours are available as needed.
Paid Holidays
Enjoy 9 paid holidays off per year.
Paid Time Off (PTO)
PTO starts accruing from the day you start.
Referral Bonus
Employees are eligible to earn a $3,500 referral bonus!
Health Insurance Plans
Enjoy a low employee co-pay with high contributions towards your plan.
Dental Insurance Plans
Yes - we have a great dental plan as well!
Quarterly Bonus
Teams achieving set performance goals receive a quarterly performance bonus.